WasteLog & Safe Label System Integration
What is Drug Diversion?
Diversion occurs when an individual in a position of trust, who is allowed legal access to controlled substances for the purpose of dispensing for approved medical uses to patients, redirects any controlled substance from the course of its legally intended purpose to an illegal purpose to include:
- Use by the individual person diverting the substance
- Use by an unauthorized third-party
- For the illegal sale/profit/trade The areas of risk for potential diversion include: procurement, preparation and dispensing, prescribing, administration, and waste and removal.
How we help solve the problem
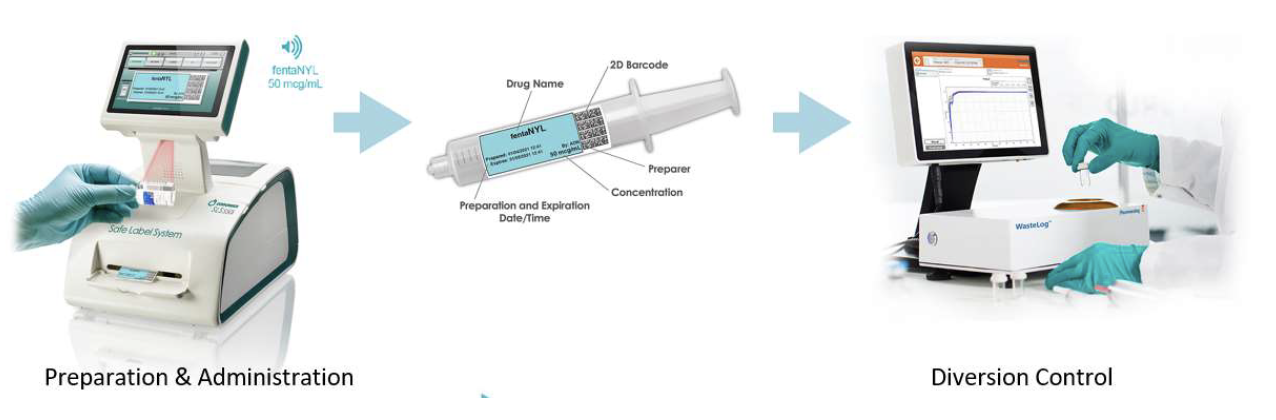
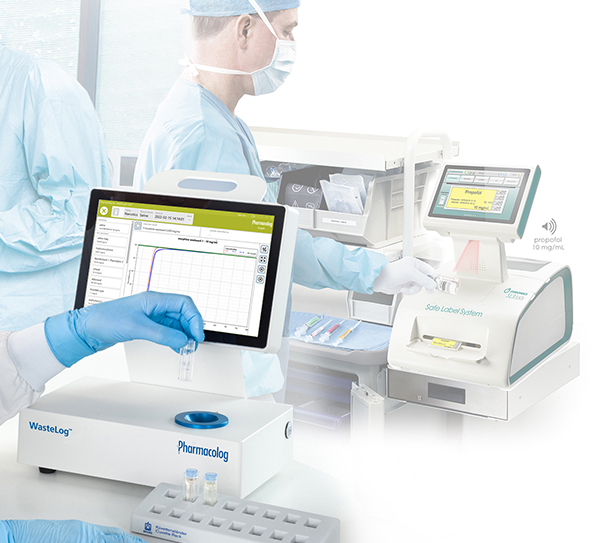
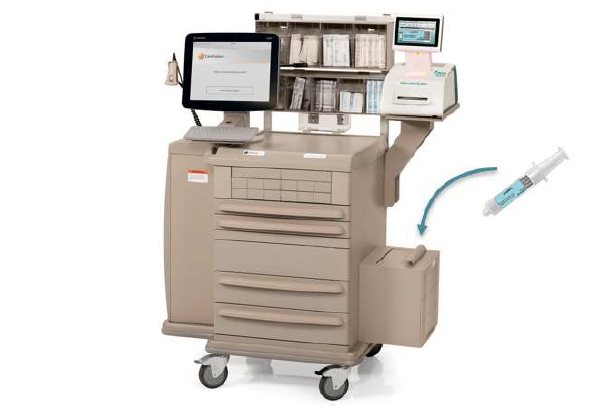
Codonics and Pharmacolog AB have entered into a partnership agreement providing exclusive sales, support and marketing in the U.S. for Pharmacalog’s WasteLog™, an efficient, affordable diversion control assay device. When integrated with Codonics Safe Label System, the combined solution provides an end-to-end diversion control system with the most efficient workflow. Together, these technologies will play a critical role in addressing the challenges hospitals face preventing diversion of controlled substances.
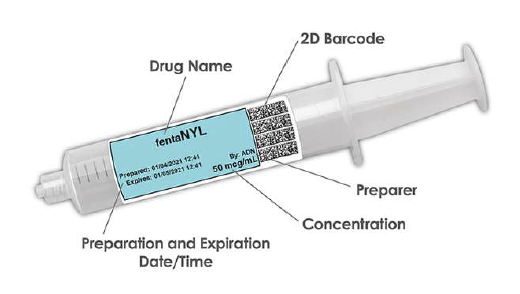
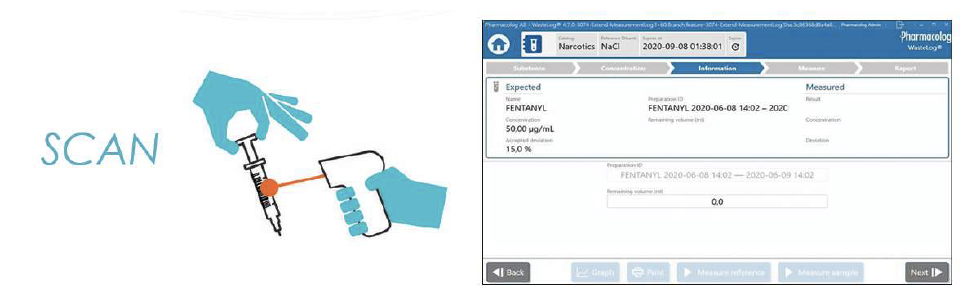
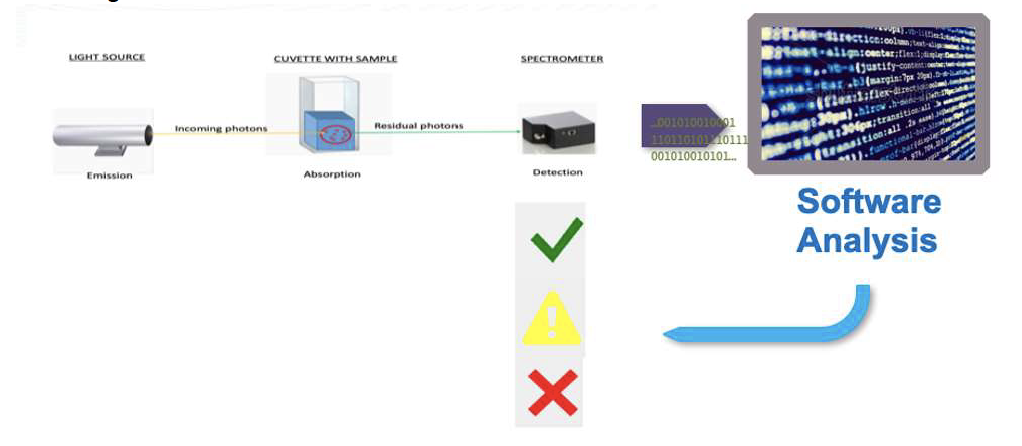
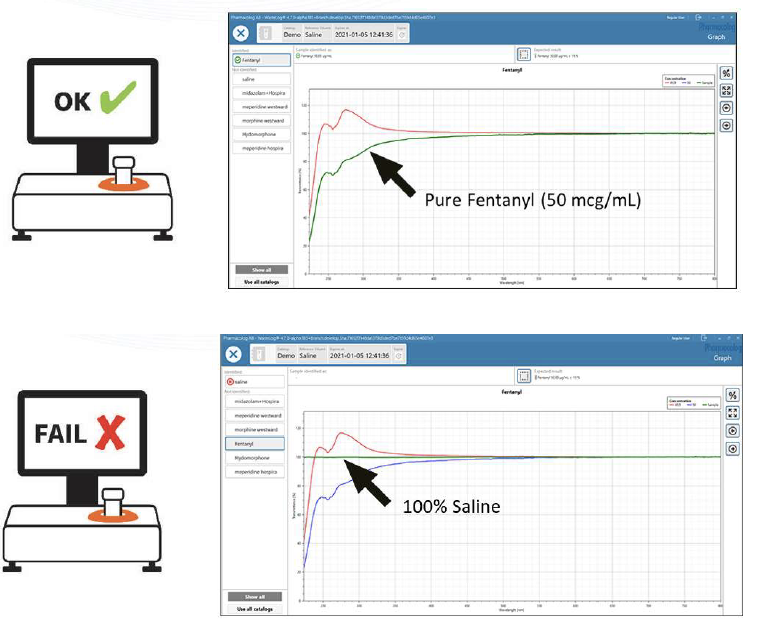
WasteLog™ is a laboratory instrument that uses UV spectroscopy technology and enables pharmaceutical waste to be analyzed and verified prior to disposal by injecting a small volume from a syringe (0.3-0.5 mL) into a cuvette to verify the contents, thereby ensuring the drug has not been tampered with. In < 3 seconds, WasteLog™ provides a robust and highly accurate result. Not only is it fast, accurate and reliable, it’s easy to use and cloud-based (with no subscription contracts) so there is no formulary management.
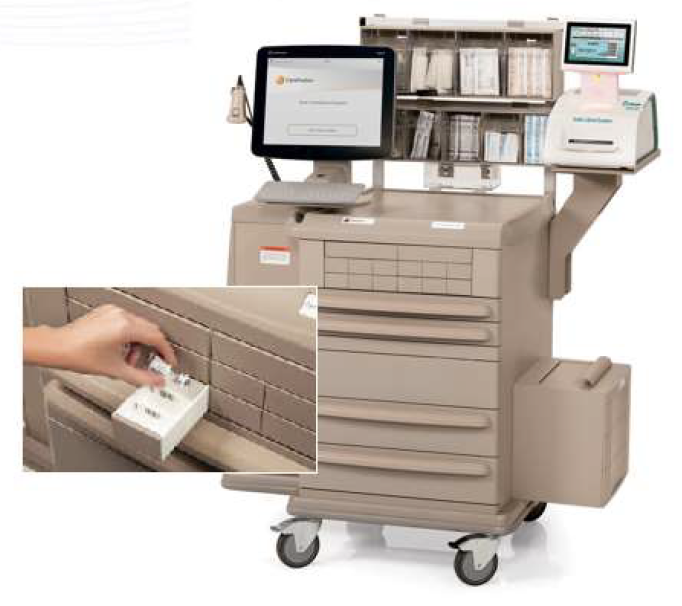
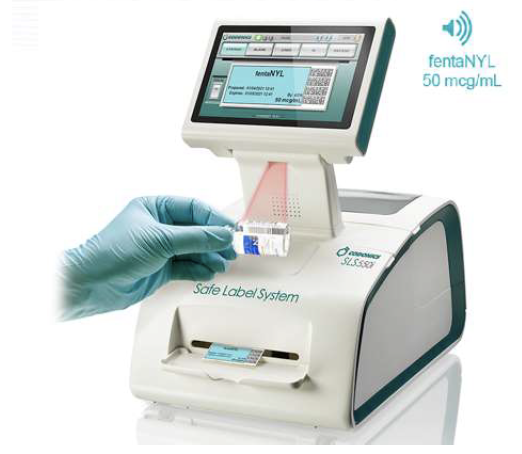
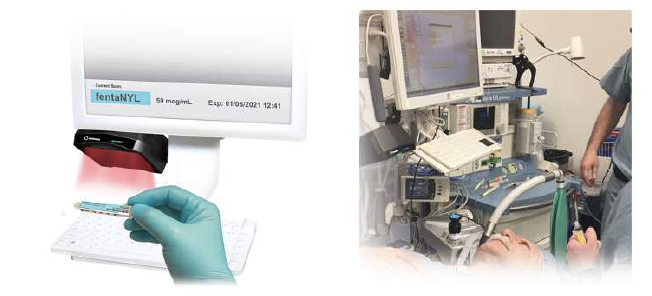
When integrated with Safe Label System, wasted and returned syringes can be scanned enabling WasteLog™ to electronically identify the drug and concentration, significantly improving the workflow. Once the drug is assayed, key data and diversion insight is quickly available for in-depth analysis and insight such as trend analysis, report generators and data filters, and can be shared with existing analytical software platforms. Plus, by using cuvettes, any sample can be sealed to preserve it further testing.
A hospital can implement Safe Label System and WasteLog™ at the same time, or may choose to implement one product at a time. When installed together, the system is:
- Integrated to combine electronic safety checks during medication preparation and administration with
- Better management of the medical waste workflow to
- Provide a robust, proven and modern approach to drug diversion